This deep dive will show you how to lower the heat, weight, and power consumption of your portable medical motion control applications and it discusses advances in the motors themselves.
Medical Devices
From blood analyzers and heart assist pumps to imaging systems and ventilators and beyond, medical device development demands state of the art performance as well as high levels of safety, stability, reliability, and long-term availability of electronic components. Further, the assurance of rigorous quality management systems such as ISO 13485:2016 and ISO 9001:2015 is of paramount importance. With decades of experience at the delicate juncture of human health and technology, PMD helps medical device OEMs and robotic engineers meet stringent industry requirements and attain long-term product success with their motion control products.
![]()
PMD is ISO 9001:2015 and ISO 13485:2016 certified. We deliver the quality, safety and long-term availability demanded by healthcare and life sciences applications.
This deep dive will show you how to lower the heat, weight, and power consumption of your portable medical motion control applications and it discusses advances in the motors themselves.
Replay the on-demand version of our popular 30-minute webinar that introduces you to the major capabilities and benefits of the N-Series ION.
Machine designers are often asked to build systems that take up less space and operate on less power. Read about new developments in motors, sensors, and architectures.










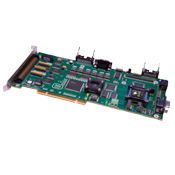
Prodigy/CME Machine-Controller boards are available in 1, 2, 3, and 4-axis configurations. They provide high performance motion control functions, on-board amplification, and Brushless DC, DC Brush, and step motor control. They allow user-written C-language code to be downloaded and run directly on the board.


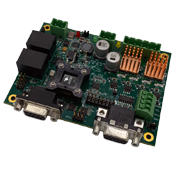
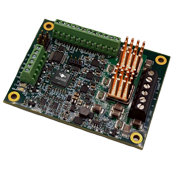




PERFORMANCE MOTION DEVICES, INC.
80 Central Street | Boxborough, MA 01719
P: 978.266.1210 | F: 978.266.1211 | info@pmdcorp.com